Uveitis
Inflammation of the pigmented vascular layer of the eye — is a medical emergency responsible for up to 20% of cases of blindness1
For patients with chronic or recurrent uveitis, whose uveitis is not controlled within 3 months of treatment with ≤10mg/day, steroid-sparing therapies have become the standard of care2.
Adalimumab approved for non-infectious uveitis fills gap3.
Dosing
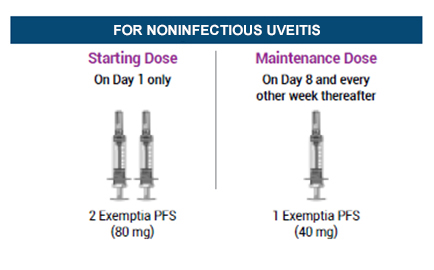
For Noninfectious Uveitis
Post Marketing Surveillance
Real world Evidences
Intermediate, Posterior and Panuveitis non-infectious Uveitis
Use, Safety and Efficacy of ZRC 3197, a biosimilar candidate of reference Adalimumab from Tertiary Pediatric Rheumatology Centre in India
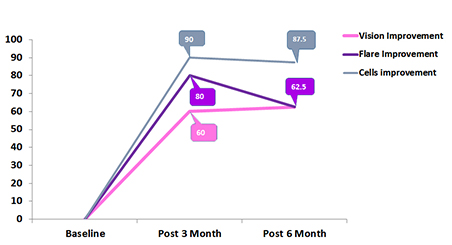
Percentage of Vision improvement, Flare improvement and cell Improvement in pediatric uveitis patents after 3 and 6 moth of follow up
Ref:
- de Smet MD, Taylor SR, Bodaghi B, et al. Understanding uveitis: the impact of research on visual outcomes. Progress in Retinal and Eye Research. 2011;30:452-570 doi: 10.1016/j.preteyeres.2011.06.005.
- Foster CS, Kothari S, Anesi SD, et al. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Survey of Ophthalmology. 2016;61(1):1-17
- Agarwal M et al. Use, Safety and Efficacy of Zrc 3197, a Biosimilar Candidate for Reference Adalimumab (Humira) from a Tertiary Pediatric Rheumatology Centre in India: Proceeding of the ACR/ARHP Annual Meeting; September 28, 2016

